CORITON completed the Eudamed manufacturer role registration under the new MDR regulations.
Editor:adminTime:2021-09-06
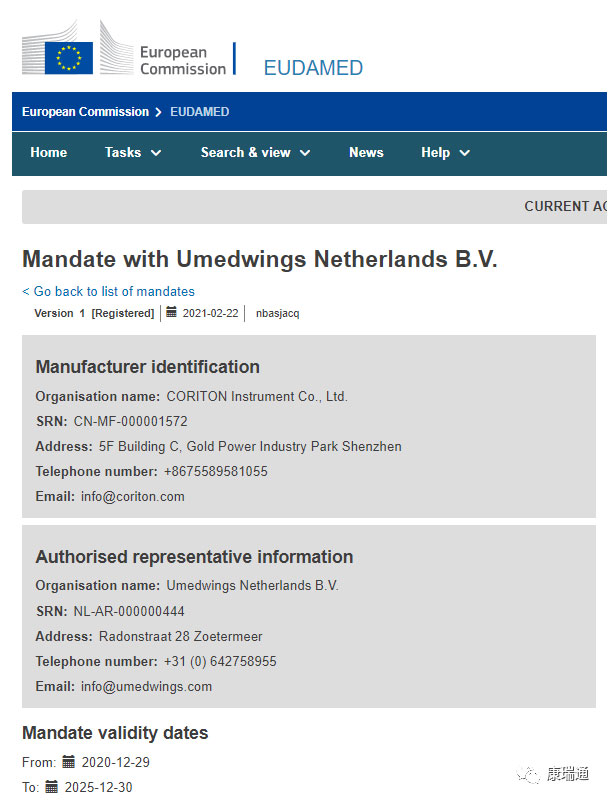
On February 22, 2020, CORITON Company completed the Eudamed manufacturer role registration under the new MDR regulations.
SRN number: CN-MF-000001572
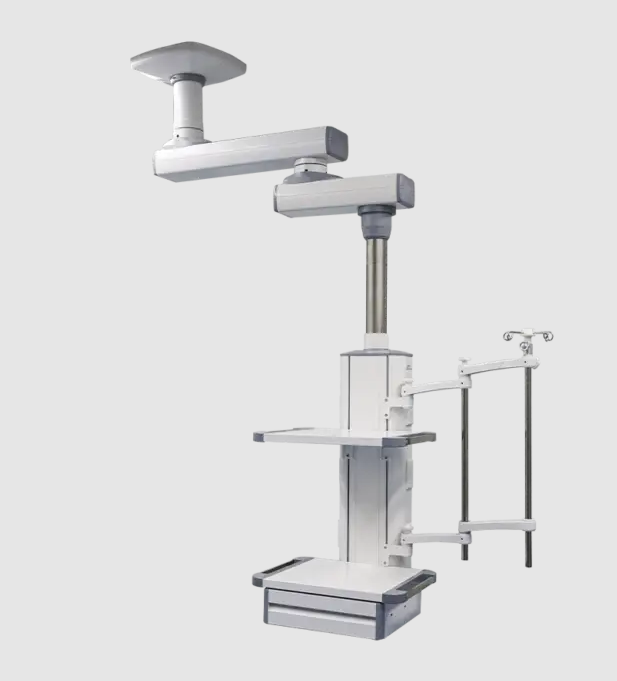
CORITON Instrument Co., Ltd. is a China leading medical trolley supplier. Founded in 2011, through years of our unremitting efforts and innovation, we have developed products that meet market needs and are of high quality and cost-effective.
The company is currently the only professional medical cart manufacturer in China that has passed the German TUV SUD ISO13485, ISO9001, and FDA listing records.
What is SRN?
The full name of SRN (Unique Registration Number) is Single Registration Number, which is the unique identification of each economic operator on EUDAMED and related official documents and reports.
What does SRN look like
The structure of the SRN code is “country code + role code + 9-digit code”. For example, “BE-MF-000000001”, the first part is the country code of the competent authority issuing SRN, “BE” here stands for “Belgium Belgium”; the second part is the abbreviation of Actor, “MF” here stands for “manufacturer” ; There are nine numbers in the third part.
Recent News
-
2024-CMEF Review: Gathering domestic and international guests, enjoying the grand event
Time:2024-04-27
-
Invitation | CORITON invites you to attend the 2024 Shanghai CMEF medical exhibition
Time:2024-04-02
-
CORITON Company will participate in Medtrade USA Exhibition, booth number: # 1027
Time:2024-03-22