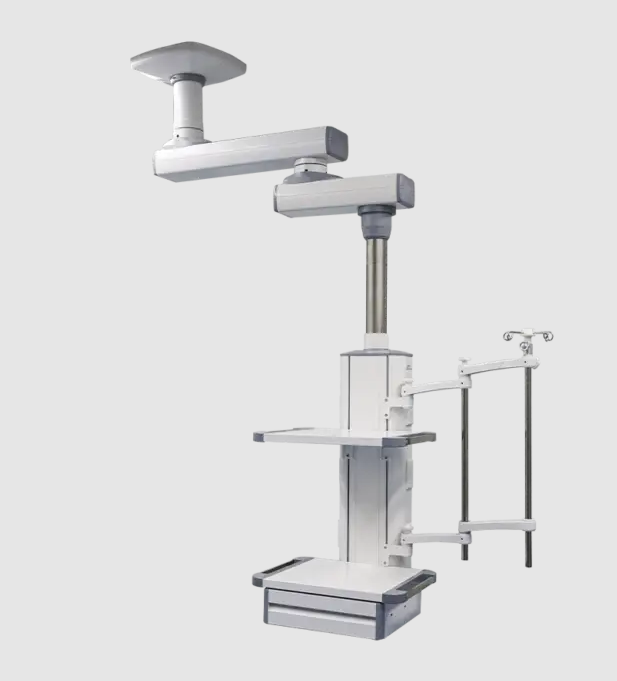
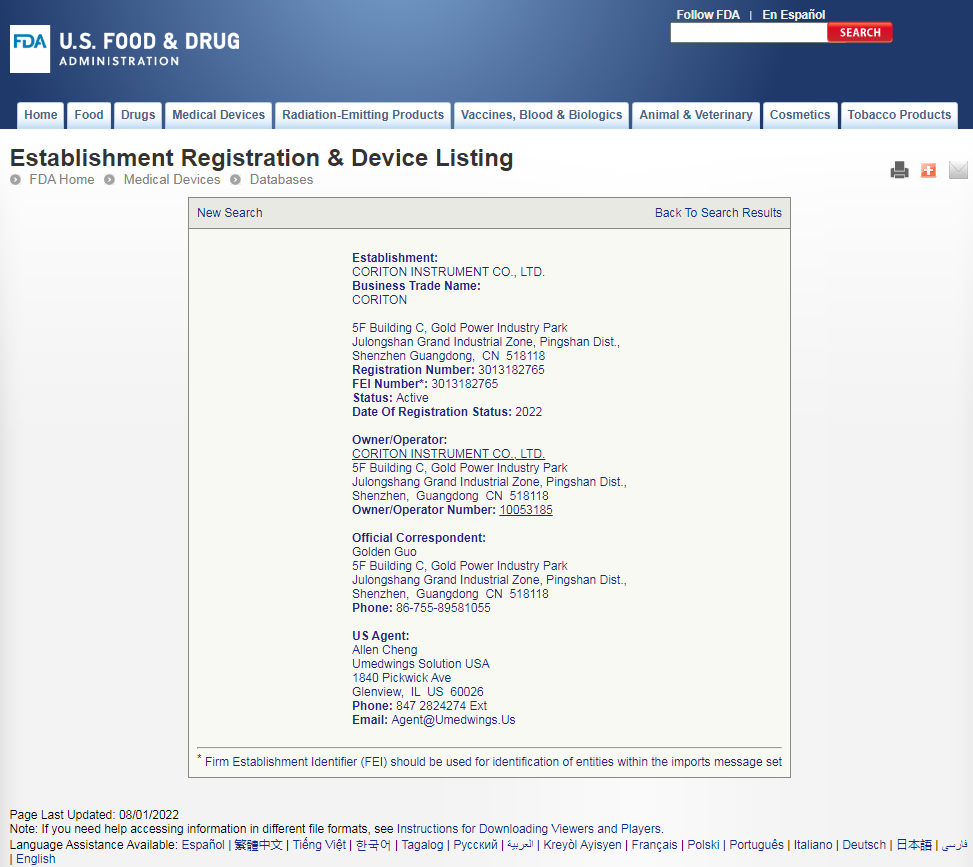
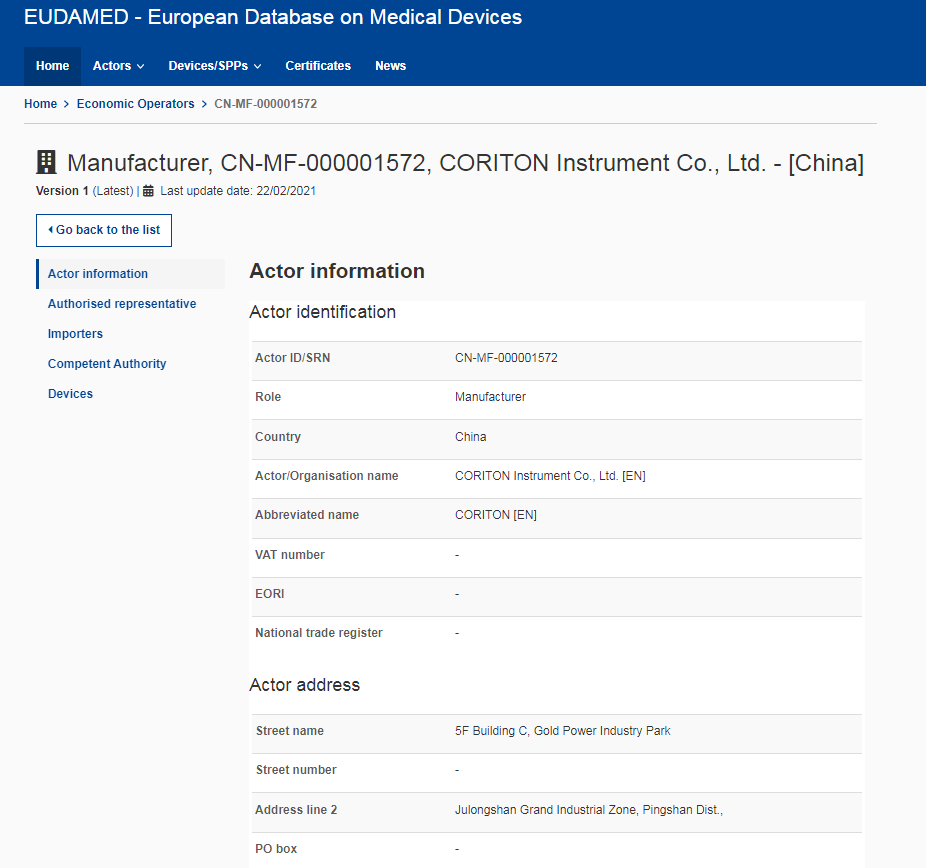
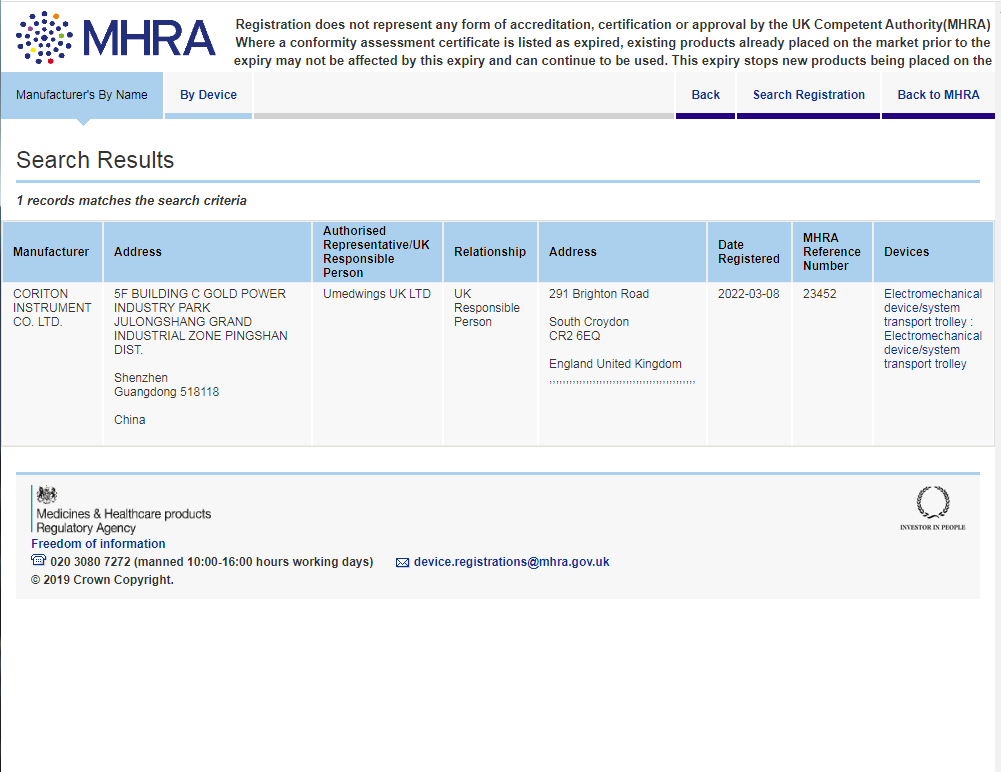
CORITON: the first medical cart manufacturer in the world to complete the simultaneous registration of the United States, the European Union and the United Kingdom
Editor:adminTime:2022-08-06
In an era of increasingly stringent global medical device regulations, the EU MDR has been implemented for a year; the UK regulations are also starting to reform, and will implement similar requirements to the EU next year.
As a leading medical cart manufacturer in China, CORITON is currently the only medical cart manufacturer in China that has obtained the German TUV SUD ISO13485 certification. The most important aspect of ISO13485 implementation is compliance with relevant regulations.CORITON has now taken the lead in completing the latest MDR CE certification in the European Union; at the same time, it has also completed the UK UKCA certification, realizing support for the regulations of the European Union, the United Kingdom and the United States.
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm?rid=200760
https://ec.europa.eu/tools/eudamed/#/screen/search-eo/2d0fd286-ddd2-4a11-a378-c300426da0e2
https://aic.mhra.gov.uk/era/pdr.nsf/Search?SearchView&Query=/era/pdr.nsf/Search?SearchView&Query=[ManName]Contains(%22coriton%22)&SearchOrder=4
Recent News
-
2024-CMEF Review: Gathering domestic and international guests, enjoying the grand event
Time:2024-04-27
-
Invitation | CORITON invites you to attend the 2024 Shanghai CMEF medical exhibition
Time:2024-04-02
-
CORITON Company will participate in Medtrade USA Exhibition, booth number: # 1027
Time:2024-03-22